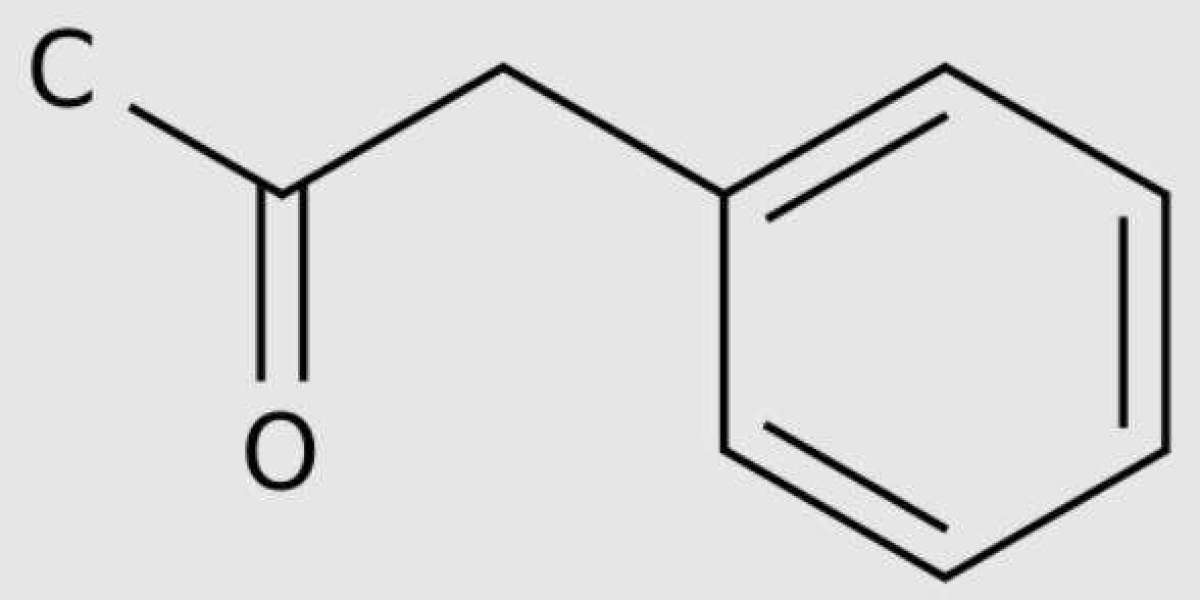
This goal is achieved by using a method of producing phenylacetone by reacting phenylacetic acid with acetic acid in the gas phase on a catalyst not lower than 350°C (the decomposition temperature of the resulting phenylacetic acid), while using cobalt oxide CoO as a catalyst on an inert support (mol.75g/mol).
The mechanism of producing phenylacetone from a mixture of phenylacetic acid and acetic acid in the gas phase is simplified to the formation of phenylacetic acid, an undetectable intermediate that breaks down under the influence of temperature into phenylacetone and carbonate of the corresponding metal (depending on the type of catalyst). This factor determines the temperature of the treatment. Us Patent No. 5,750,795 states that the process temperature shall not be lower than 250°C in the case of catalysts containing magnesium oxide and 450°C in the case of catalysts containing calcium oxide.
As the authors suggest, over time a decomposition balance is established between the metal oxides in the system and their carbonates. If these temperatures are not observed, the entire catalyst will become carbonate and thus deactivate. This explains the lack of activity of barium oxide due to the high decomposition temperature of its carbonates (1450°C), despite being an alkaline earth metal. This argument does not make sense. In the present invention, cobalt oxide is used as a catalyst where the temperature of carbonate decomposition to oxide is 910°C, but the production reaction of phenylacetone is still ongoing and the yield exceeds the yield in the above-mentioned patent. It can be seen that this method can also occur when only metal carbonate is present as the catalyst.
Cobalt oxide shows a higher activity than the catalyst described in the above patent. The decomposition process of acetate was studied by derivatization method. It is worth noting that symmetrical cobalt acetate (acetic acid and phenylacetic acid) has the closest decomposition temperature relative to other types of catalysts, i.e. these substances are similar in terms of alkalinity. Possibly, this factor also affected the mixture of phenylacetate, resulting in the formation of phenylacetone relative to acetone and dibenzyl ketone.