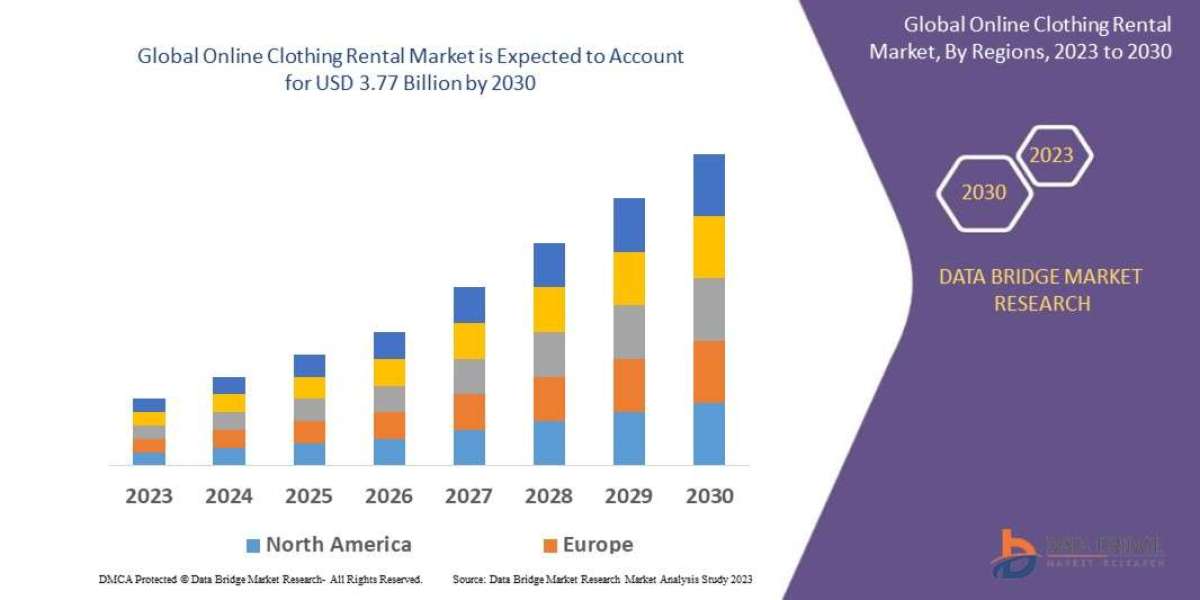
Objective: To determine the efficacy of serum total testosterone levels (TT) and luteinizing hormone (LH) in men with secondary hypogonadism following initial and long-term daily oral enclomid citrate compared with transdermal testosterone School (PD) status. To determine the effect of daily oral doses of enclomid citrate (Androxal®) compared with transdermal testosterone on other hormones and markers in men with secondary hypogonadism.
PATIENTS AND METHODS: This was a randomized, single-blind, two-center phase II study evaluating the efficacy of three different doses of enclomid citrate (6.25 mg, 12.5 mg, and 25 mg Androxal®) versus AndroGel® (a A transdermal testosterone) within 24 hours. LH and TT hours in otherwise normal healthy men with secondary hypogonadism. Forty-eight men participated in the trial (ITT cohort), but 4 men had baseline T levels >350 ng/dL. Forty-four men completed the study per protocol (PP population). All subjects enrolled in the trial had serum TT in the low range (<350 ng/dL) and LH in the low to normal range (<12 IU/L) on at least two occasions. TT and LH levels were assessed hourly over 24 hours to examine the effects of three therapeutic doses of Enclomid versus the standard dose (5 grams) of transdermal testosterone (AndroGel). In the initial curve, TT and LH were measured in the initial population after the initial oral or transdermal treatment (day 1). This is in stark contrast to what was observed after six weeks of continuous daily oral or transdermal treatment (day 42). The pharmacokinetics of Enclomid was performed in selected subpopulations. Serum samples were obtained during the study to determine levels of various hormones and lipids.
RESULTS: After six weeks of continuous use, men taking the highest dose of androxal enclomid citrate (enclomid, 25 mg per day) and 500 ± 278 ng had a mean ± SD TT concentration of 604 ± 160 ng at C0hrTT on day 42 /dL. Among men receiving transdermal testosterone. These values were higher than those on day 1 but were not different from each other (p = 0.23, T-test). All three doses of Enclomid increased C0hrTT, CavgTT, CmaxTT, CminTT and CrangeTT. Transdermal testosterone also increases TT, albeit with greater variability and suppressed LH levels. The TT pattern within 24 hours after six weeks of dosing may fit a non-linear function of morning rise, midday trough, and nighttime rise. Enclomiphene and transdermal testosterone increased TT levels over two weeks but had opposite effects on FSH and LH. Enclomiphene treatment did not significantly affect levels of TSH, ACTH, cortisol, lipids, or bone markers. Both transdermal testosterone and enclomid citrate decreased IGF-1 levels (p<0.05), but the inhibitory effect was greater in the enclomid citrate group.
Conclusions: Enclomid citrate increased serum LH and TT; however, there was no temporal association between peak drug levels and Cmax levels of LH or TT. Enclomid citrate consistently raised serum TT to the normal range and raised LH and FSH above the normal range. The effects on LH and TT persisted for at least one week after stopping treatment.